Biomarin: a study of a public relations nightmare in the making
Today I examine a company who not only serves as an example of the ethical imperative in the structure and design of a business, but I may even be able to forecast a crisis in the making. Most case-studies of crises occur after a crisis has ended or at least after it has begun, but the actions of the pharmaceutical company BioMarin over the last several weeks – while not reaching crisis yet – are moving quickly in the direction of one.
The background of the story is that BioMarin has created a drug called BMN673 that has not yet been approved by the Food and Drug Administration (FDA), but which has shown enormous promise to patients who have gone through trials of the drug. Between 5-10% of ovarian cancer patients develop the cancer due to a genetic mutation called BRCA1. This drug was created for that rarer set of ovarian cancer patients. Creating drugs for rarer diseases is BioMarin’s specialty.
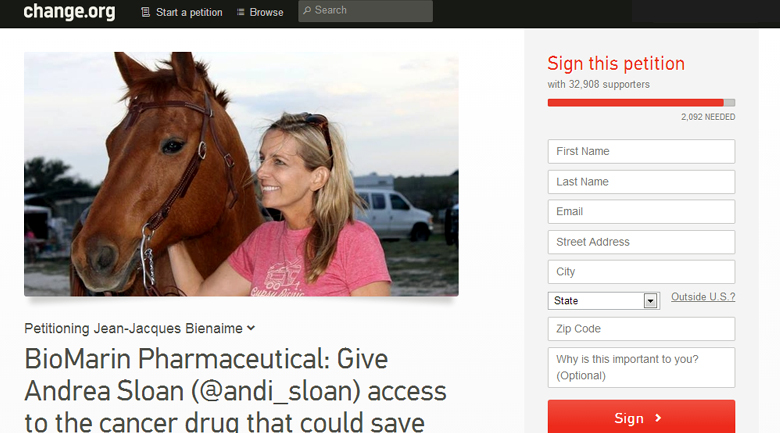
A young attorney in Austin, Texas named Andrea Sloan has the BRCA1 mutation and has fought ovarian cancer off and on since 2007. Traditional treatments are failing at this point, and Andrea’s doctors at MD Anderson view BMN673 as Andrea’s best and possibly last hope.
BioMarin designed this drug for Andrea Sloan, but so far, they will not let her have it. They respond to her requests for the drug by saying that they simply do not have a policy for compassionate use of this drug and it is too early to give out. The FDA, though, has confirmed that it will provide a waiver for Andrea to have access to the drug immediately if the company will only agree to give it to her.
While BioMarin tells Andrea that they want to wait longer to know the drug is safe, the company has issued scientific reports and the CFO has addressed investors with news that the drug has worked well in patient trials and that patients have tolerated the drug very well.
The crisis isn’t over yet
Alert to all crisis management consultants: BioMarin is a company that is not normally public facing yet who may soon face very public scrutiny as a symbol of a broken system
BioMarin’s own “Global Code of Conduct and Business Ethics” states: “It also includes embracing and creating innovative and ethical programs and strategies for expanding patient access to BioMarin products designed to meet unmet medical needs, while at the same time ensuring an appropriate return for our shareholders.”
In pharma-speak, “patient access” often seems to mean “compassionate use” or use as a last resort or under special circumstances. The company has created a crisis precursor for itself by publicly promoting the efficacy of this particular drug to investors while denying its use to a patient who has the exact problem for which it was designed. The one qualification they allow themselves in denying such use in their own rules is “ensuring an appropriate return for our shareholders.” Even though the FDA has indicated it will approve Andrea’s use of the drug, the company has apparently decided that providing the compassionate administration of the drug is somehow not in their financial best interest.
There are ethical obligations the company has to its shareholders, but one should not assume that the shareholder’s only concern is short-term financial. Even if that is the primary concern, the negative public scrutiny that could result from denying this patient the drug could easily harm both the short- and long-term economic health of the company’s publicly traded stock $BMRN.
Contradictory statements and actions
Even if BioMarin’s CFO was not touting the value of BMN673, its reports to the scientific community about the extraordinary potency and the ability for patients to tolerate the treatment provide an ethical obligation for the company to make the drug available to a person who needs it as a last resort.
We have a situation where a drug company has created a drug that is still in trials, but has shown tremendous promise. We have a regulatory body which has shown approval for use of the drug for compassionate use. And we have a patient who has the exact problem for which the drug was created and who understands and accepts the risks inherent in taking a drug that has not yet completed trials.
By what ethical standard can BioMarin continue to deny Andrea Sloan this drug which she must get within the next several days? A moral imperative should supersede a financial one when a person’s life is on the line, but how would BioMarin even face a financial risk in allowing this compassionate use of their innovative drug?
Social media accelerates attention to this issue
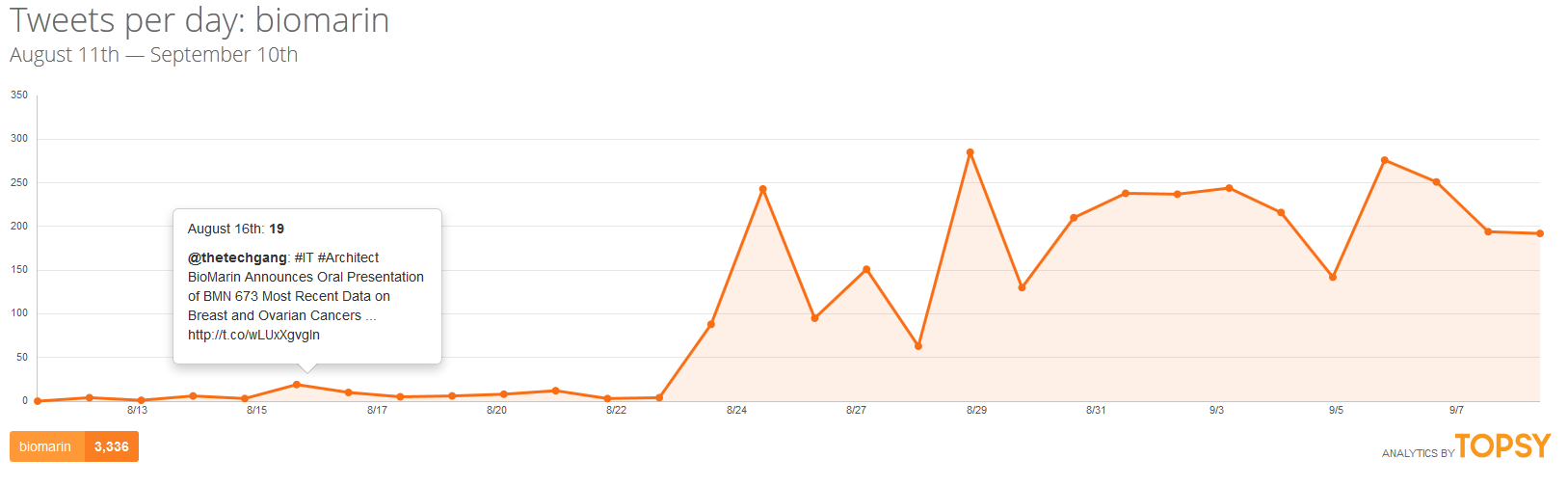
If all of this was happening in a vacuum, there might be little risk of the company facing a crisis. But in today’s atmosphere of social media saturation blending into traditional media coverage, this company which normally only communicates with scientists and investors may find itself as the face of a broken system and be held accountable by the media and a public who might not have the most favorable view of pharmaceutical companies already.
Supporters of @andi_sloan have already initiated a social media campaign that has gained the attention of many people who have never met Andrea Sloan, including a number of celebrities and political figures. A petition has circulated that has already received support of over 30,000 people. CBS This Morning has been the first national media outlet to cover the story and in that story, BioMarin showed that it is not yet ready to face media scrutiny judging from its avoidance of commenting on the issue.
BioMarin can still decide to give Andrea Sloan access to the medication which is sitting on a shelf at the MD Anderson Cancer center where Andrea Sloan receives treatment. If they continue to refuse, however, the company is opening itself to potentially severe scrutiny that could easily rise to the level of a crisis for the company.
David Holmes, owner of Intrepid Solutions, has over 20 years experience planning for, avoiding, and solving crises in the public policy, political, and private sectors. David is also a professional mediator and has worked in the Texas music scene.





































Lani Rosales
September 12, 2013 at 3:15 pm
I just had an interesting conversation with a specialist in this field who cannot comment on the record but offered the following (sad) clarification:
“Your writer’s piece is thoughtful and I agree with all of his points with regard to a PR crisis in the making. But he seems to lack an understanding of how pharmaceutical companies make decisions. Compassionate use is never about one patient and by providing an unproven drug to one patient, a company could risk billions of dollars. It seems inhumane but pharmaceutical companies toe the line between treating disease and running a business. Apparently, they offered to insure her place in a clinical trial; the caveat lies with when that trial will start.”
David Holmes
September 13, 2013 at 2:23 am
No, anonymous. I lack no understanding of how radical capitalists make decisions. Compassionate use is NOT about one patient and that has never been the tilt here. BioMarin would not risk one dollar in providing an individual or several individuals with FDA approved access to their treatments. Where this anonymous contributor seems to lack understanding is that BioMarin has been publicly (and possibly criminally) promoting the efficacy and tolerability of this drug; inflating its stock value; selling a few million dollars of stock off the inflated price based upon their testimony of the value of the drug in the last few days – all while saying the opposite to the one patient who could benefit from their government-funded-inflated, orphan drug. (another thing Anonymous appears to have little if any knowledge of is orphan drugs. The whole reason this drug exists is because there are not many people who can benefit from it; much less lines of people awaiting trials.)
Let me repeat the important part of that. While Anonymous opines about an “unproven” drug; BioMarin executives have promoted the drug by illustrating over the last couple weeks that it is 200-times more potent than any drug on the market. That means – and I did not understand this without it being explained to me – that a patient can tolerate the drug at doses 200 times smaller than drugs currently on the market. Read just one of their scientific reports on the drug and there is no adverse reaction they will admit to beyond minor, discomfort far less than the average drug in this category. Everywhere the executives of this company go, they talk about how amazing this drug is; except in response to patients to whom they say it is unproven.
So, again, I lack no understanding in how pharmaceutical companies make decisions. It is sick and it should change and whether or not she can impact this company in a manner that will prolong her own life, Andrea Sloan has been and will continue to fight so that others will not face this same bullshit.